Cell and Gene Therapy
Cell and gene therapies are growing at a rapid rate and Almac recognizes that with these positive advancements come a series of potential new challenges for all sponsors.
The accountability and reconciliation process is noted for being time-consuming and error-prone, two characteristics that result in it adding significantly to trial costs and timelines. The onerous task of reconciling discrepancies in supply records that have accumulated over the course of a trial often adds as much as two years to the study closeout phase.
We have an in-depth understanding of the emerging challenges facing sponsors of next generation biotherapeutic trials. Harnessing over 30 years’ experience, advanced technology and global infrastructure, we customize comprehensive supply chain solutions. We put patients at the heart of all decisions, restore order & cost control, safeguard integrity throughout product lifecycle and lay the foundations for successful commercialization.
Effective strategies to safeguard and streamline
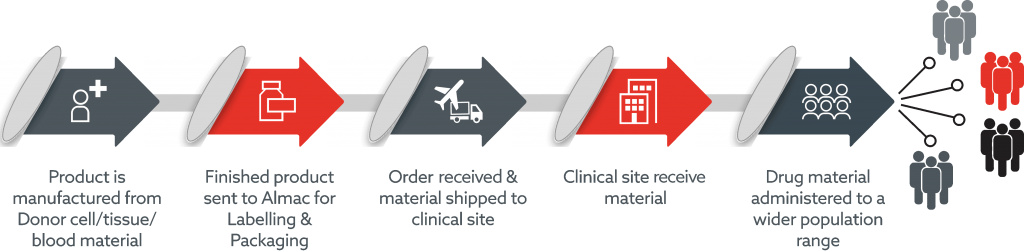
Advanced therapy supply chains are particularly complex with many moving parts. From initial patient contact to logistics, production, distribution and patient administration, Almac’s team of dedicated experts are uniquely positioned to design and operate effective supply chains across all realms.
Effective supply chain strategies to safeguard and streamline:
- Provide valuable insight to predict the unpredictable achieving scalable, patient-centric results for sponsors
- Combining consultative expertise with fully integrated forecasting technology to minimize waste and control costs
- Complete supply chain control – minimizing excursions, maximizing compliance and safeguarding supply to sites and patients


Thinking outside of the box with innovative production
Through integrated manufacturing, expert data management and strategic logistics, we create optimized operations that reduce risk and minimize waste.
Packaging strategies that adapt to the challenges of your trial:
- Adaptive, patient-centric and on-demand packaging capabilities
- Development of innovative and personalized clinical packaging and labeling strategies for sponsors designed for next generation biotherapeutics
- Fully validated processes that uphold compliance and safeguard integrity for sensitive, high value products like cell and gene therapies designed for global distribution


Assuring products’ integrity through the cold chain
To manage the additional complexity global distribution of cell and gene therapies presents, we have combined specialist people, processes and technology to offer a robust, temperature management distribution package that can be adopted to suit varying needs.
Assuring products’ integrity through cold chain logistics:
- End to end regulatory compliant temperature management solutions that give full visibility of a product’s temperature data from any stakeholder within the supply chain
- A robust network of reliable couriers combined with over 60 strategically positioned global deports to provide global coverage for clinical trial distribution
- Risk based management strategies through Almac’s control tower to provide import/export, courier management and shipping lane data analysis to ensure your cell and gene product gets to the right place, at the right time and in the right condition


Almac’s cell and gene therapy suite of services
Allogeneic Therapy – Patient kit manufacture & logistics