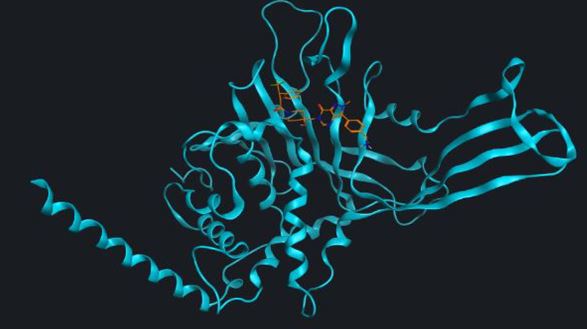
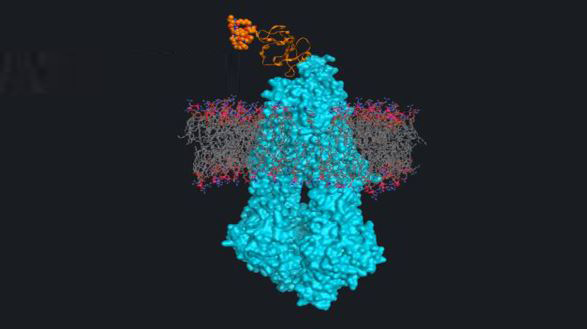
Pipeline
We are a research-driven biotech company dedicated to the discovery of first-in-class therapies addressing unmet needs. Our platforms and portfolio deliver NCEs and protein therapeutics against novel targets of relevance in oncology and neurodegeneration.