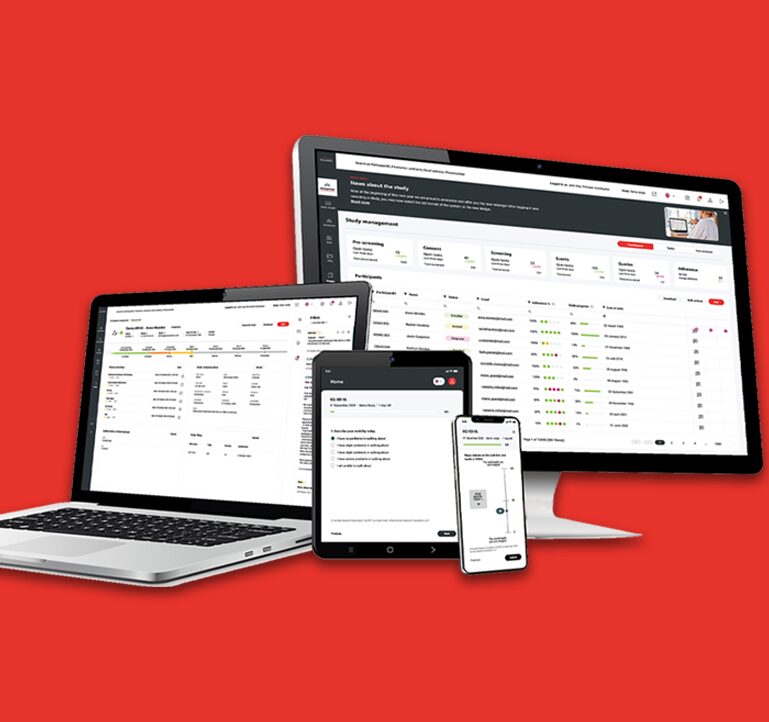
A fully Integrated eCOA Platform for Streamlined Clinical Trials.
Almac Clinical Technologies offers a fully integrated eCOA platform that streamlines clinical trial operations, ensures compliance, and enhances the experience for participants, sponsors, and sites. Designed for flexibility and global scalability, our solution simplifies outcome data collection and supports real-time, data-driven decision-making.
Built on Almac’s legacy of regulatory excellence and innovation, the platform is multilingual, configurable, and seamlessly integrates with our industry leading IXRS®3 IRT system. Whether used independently or as part of the Almac ONE™ ecosystem, it delivers high-quality, real-time data to power confident trial decisions.
Download eCOA Factsheet
Almac eCOA Resources
Explore our extensive library of resources on Almac’s integrated eCOA management software, designed to support all stages of your trial.