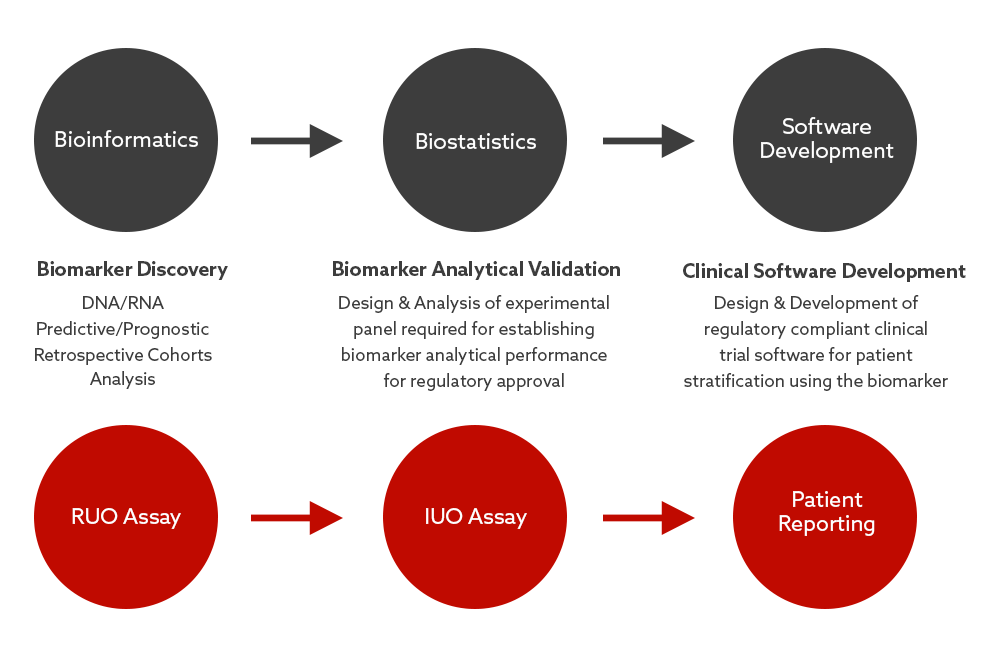
Our Bioinformatics, Biostatistics & Software Development team capabilities complement our wet lab operations in every aspect of supporting biomarker programmes, as well as providing stand-alone technical services.
At each stage of development or delivery of a biomarker, we have established dry lab processes in place to maximise the successful development & delivery of the clinical trial assay (RUO, IUO or CDx). Analysis is standardised and repeatable, and rigorously tested & validated in accordance with regulatory standards.
The team spans three regions (UK, EU and USA) and is comprised of highly experienced scientific experts
Our range of services:
Click each link below to find out more information about our Bioinformatics, Biostatistics and Software Development capabilities:
Almac proprietary analysis tools for better client data insights:
Almac Diagnostic Services also invests R&D time into proprietary bioinformatics products to enhance the data output of sequencing and analysis for biomarker discovery.
Benefits of Almac Data Sciences approach:
Having these capabilities available all in one location, through an experienced service provider means a more efficient and streamlined service for clients transitioning a clinical assay from the initial discovery phase through to clinical delivery.
Each stage of the process is designed to ensure subsequent stages are entered into with minimal risks and obstacles.
Data Sciences supporting diagnostic development: