Almac’s Flow Assisted Synthesis Technology platform has developed significant expertise in four key areas:
- High Pressure
- High Energy
- Oxidation
- Photochemistry
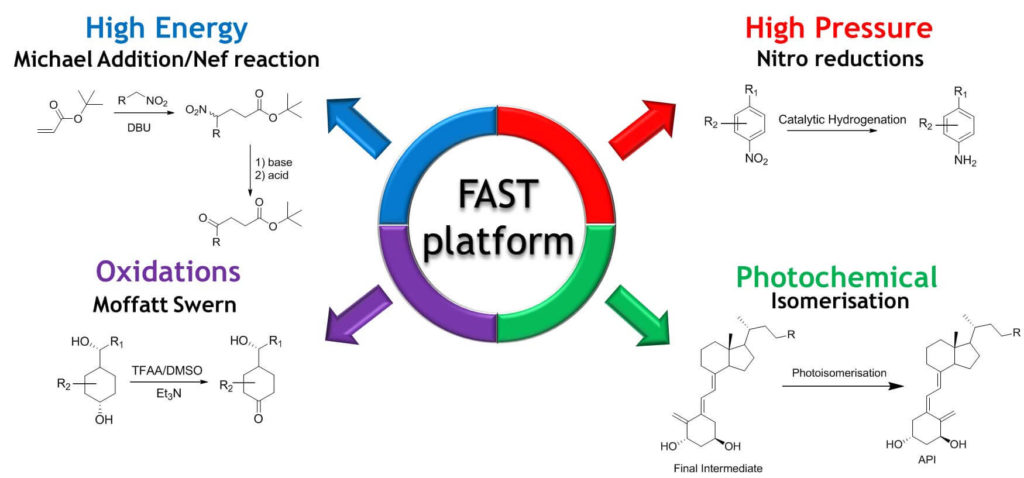
Almac continues to drive innovation within their Department of Technology with additional focus on cannabinoids and flow biocatalysis. Multiple grants support our research platform which allows us to undertake high risk but high reward projects including a Knowledge Transfer Partnership and three Horizon 2020 PhD’s with Queen’s University Belfast and a Science Foundation Ireland Industry Fellowship in collaboration with University College Dublin.