DDIR (DNA Damage Immune Response)
Almac DDIR – A predictive signature for DNA Damaging Agents and Immuno-Oncology
Almac has developed a 44 transcript RNA based gene expression signature (formerly known as DDRD) that detects a subgroup of tumours that have activation of immune signalling in response to genomic instability/ associated with loss of the Fanconi Anaemia (FA)/ BRCA pathway.
The Underlying Biology
The DDIR molecular subtype represents tumours with a loss of function of the FA/BRCA pathway, a key response mechanism to stalled DNA replication in the S-phase of the cell cycle. A loss in the FA/ BRCA pathway or exogenous DNA damage can cause an accumulation of the cytosolic DNA sensor cGAS, activating the STING/TBK1/IRF3 innate immune response. Constitutive activation of the cGAS pathway stimulates the immune transcription of chemokines CXCL10 and CCL5. Consequently, an infiltration of interferon-related genes including T-cell specific ligands such as CD4+ and CD8+ are seen in the epithelial region of DDIR positive tumours.
Application
The DDIR signature can be applied to formalin fixed paraffin embedded (FFPE) tumour specimens across multiple disease indications (breast, ovarian, oesophageal, prostate and others). Discovery and validation of this signature has been published in various scientific journals. (See related resources below).
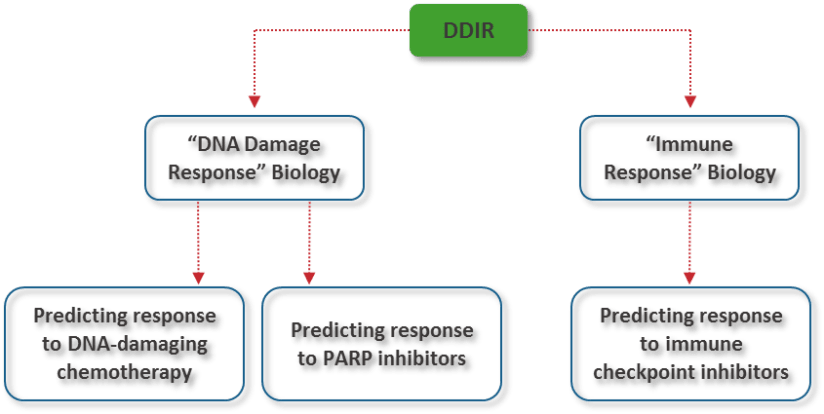
Considering the underlying biology of the signature, DDIR branches into two main utilities:
Almac’s DDIR signature has the potential to be used as a companion diagnostic for either utility across a range of disease areas.
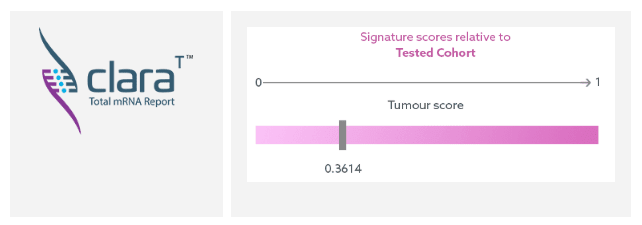
Assessment of the Almac DDIR Signature can be made through use of Almac Diagnostic Services unique gene expression report, claraT. This provides visualisation of the DDIR signature scores as an easy-to-interpret graduated bar within the Avoiding Immune Destruction and Genome Instability & Mutation Hallmarks of Cancer.