The Dashboard:
Almac Diagnostic Services has developed a dashboard report designed to allow clients access to key information regarding their clinical studies.
The dashboard displays key data corresponding to client clinical studies, including:
• Clinical Trial Summary – Received & Reported
• Clinical Site Summary
• Clinical Trial Status
• Test Results
• Clinical Trial Inventory
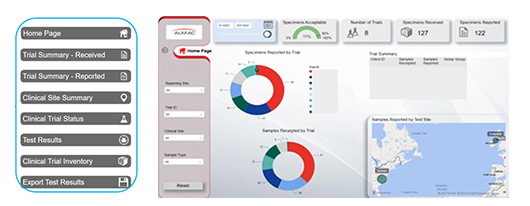
Report Tab Almac Clinical Trial Dashboard Home Page
NOTE: The data contained within the report is intended for informational purposes only and is not intended to be used to inform subject treatment decisions.
Benefits Include:
- Clients can access information on clinical study performance: quality, turnaround time (TAT), clinical site metrics and biomarker data.
- Dashboard updates automatically on a daily basis offering the most up to date information for clients.
- Clients can view clinical sample information for their studies to determine current inventory at Almac labs.
- Clients can interrogate and export multiple clinical trial data options for more in-depth queries and analysis.
Screenshots:
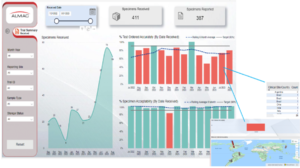

Clinical Trial Summary – Received & Reported
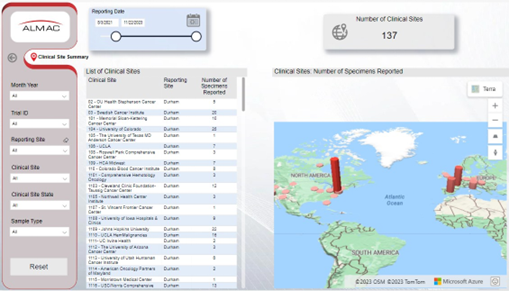

Clinical Site Summary
Fact Sheet:
Almac Diagnostic Services Clinical Trial Dashboard:
For further information download the Almac Diagnostic Services Clinical Trial Dashboard fact sheet.
Sign Up Process:
You must be an Almac client with an existing clinical trial to avail of this service. Speak to a member of the Almac Diagnostic Services Business Development Team to understand more on how to sign up to the Clinical Trial Dashboard.



