IVDR – Background
Medical technologies are tightly regulated in the European Union.
Previously, under IVDD guidelines – Roughly only 20% of devices were certified by a notified body with a CE mark on market. Now, under the new IVDR regulation – This number increases dramatically and more than 80% of all new devices will need certification by a notified body to obtain a CE Mark and be placed on market.
Before an IVD (in-Vitro Diagnostic) can be legally placed on the EU market, a manufacturer must comply with the requirements of all applicable EU legislation and affix a CE mark to their device.
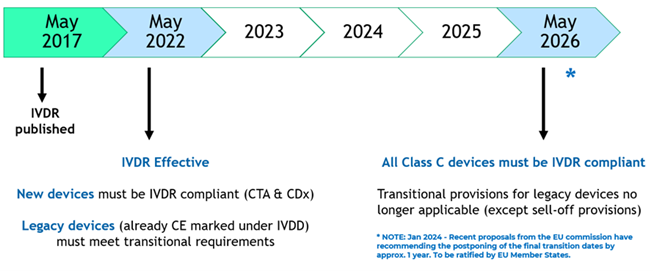
IVDR – Impact for Biopharma Companies & Clinical Trials
Recently issued EU guidance on the interface between the Clinical Trial Regulation (CTR) and IVDR places increased responsibility with pharma sponsors for the oversight, safety and performance of tests selected for use within drug clinical trials by the trial sponsors.
This guidance obligates the sponsor of drug clinical trials to obtain a statement of compliance to IVDR’s general safety and performance requirements (GSPR) from manufacturers of IVDs for use within clinical trials.
Additionally for unapproved tests utilised in an interventional manner (e.g. where treatment arm is being determined by the test) or as performance devices where the device is under investigation, the IVD manufacturer will be required to obtain clinical performance study (CPS) regulatory approval for use of their test from each EU Competent Authority in which the clinical trial is active.
Almac IVDR Support for Biopharma Companies
Almac Diagnostic Services can support Biotech and Pharma companies with up-to-date guidance and support for your biomarker programmes around IVDR and compliance with the new regulations for Europe.
- Almac is well positioned to meet the requirements of IVDR to provide services that allow the development, validation and delivery of clinical trial assays for analysis of specimens from EU clinical trial sites.
- Almac can ensure that pharma clinical trial sponsors meet their obligations under CTR.
- Almac can ensure that not only EU requirements are met, but additionally for our Biopharma partners global trials, that all other regulatory requirements are aligned optimally for smooth IVD approval in each region.
Almac IVDR Preparedness
- Almac has reviewed the appropriate IVDR Regulation.
- Almac has updated our procedures and documents as part of our QMS to ensure compliance, including PMS (Post Marketing Surveys) & reporting, GSPRs (General Safety & Performance Requirements) and Scientific Validity.
- Almac has already transitioned some CE Marked assays from IVDD to IVDR to allow use within clinical trials within the transition period by our Biopharma clients.
- Almac are currently in the process of submitting Clinical Protocol Study (CPS) applications (on behalf of major Pharma & Biotech partners) for current assays to enable use of data for registrational trial purposes including:
- Spain
- Portugal
- Germany
- Turkey
Almac Unique Geographical Location & IVDR Benefits
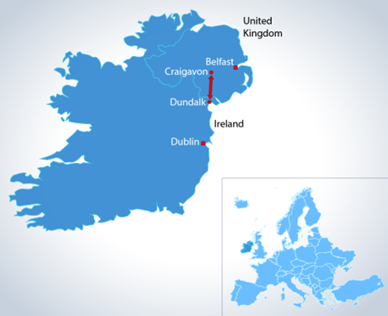
Northern Ireland is the only part of U.K. to have a land border with the EU. Almac Diagnostic Services, is in the unique global position of being based in Northern Ireland, which, due to the Windsor Agreement, has unfettered access to both the EU and UK Markets.
The UK Government has guaranteed unfettered access for Northern Ireland’s businesses to the rest of the UK internal market. For medical devices, this means that any CE marked device held by a Northern Ireland business is valid for the whole of the UK market provided it falls within the definition of a qualifying “Northern Ireland good”. Therefore, CE marked devices that can be placed on the Northern Ireland market and are qualifying Northern Ireland goods, can also be placed on the UK market and will not need to undergo any further registration in Great Britain.
This places Almac in an extremely advantageous position for assay development and deployment in clinical trials, saving time, costs & additional regulatory hurdles along the approval process for assays approved from our Craigavon laboratory.

Almac Diagnostic Services
Blog – ‘Almac Voice’
Read our latest blog on “Potential impact of IVDR on diagnostic testing in Europe – What are the implications for the pharmaceutical industry?”
Written by Joe Clune, CDx commercialisation consultant and Stewart McWilliams, VP of Quality and Regulatory Affairs at Almac Diagnostic Services, the blog covers the following key topics:
- Introduction to IVDR
- IVDR – What’s New?
- Challenges in the implementation of IVDR
- Background – Companion Diagnostics
- The evolving landscape of approved tests vs LDTs
- Will Europe still have LDTs with IVDR?
- What does Pharma need to do?
Almac Webinar – Available on demand
In this Almac webinar, hosted by X Talks, the expert speakers focus on their experience with IVDR and describe strategies for solving the specific challenges faced during the IVDR submission process for clinical trial assays being utilised as CDx, allowing global trials to commence and complete in a timely manner.
Download this webinar today where experts will share their experience with EU IVDR and provide insights into solving challenges faced during the EU IVDR submission process.
Presenters:
Dr. Stewart McWilliams, Global VP Quality and Regulatory Affairs, Almac Diagnostic Services


Charlene Robb, IVD Regulatory Affairs Team Leader, Almac Diagnostic Services


Contact us
Looking for an informal conversation on how Almac Diagnostic Services might help with your IVDR requirements?
Get in touch and one of our experts will follow up with you.
Contact us




