Almac Diagnostic Services Craigavon and Durham laboratory sites hold multiple accreditations and laboratory permits in compliance with various regulations as outlined below.
Our Approach
The Almac Quality Management System (QMS) is managed by a multi-disciplinary team including experts in Quality Assurance, Quality Systems, Validation & Regulatory Affairs. Our QMS covers the design, development and manufacture of in vitro diagnostic (IVD) devices, in addition to the quality requirements of the clinical testing laboratories which includes all aspects of the pre-analytical, analytical and post analytical phases of testing to ensure patient safety goals are addressed.
UK Laboratory Site (Craigavon, NI)
| Accreditation / Licence / Permit | Certificate Number | Authorising body |
| Clinical Laboratory Improvement Amendments (CLIA) | 99D2017022 | Centers for Medicare & Medicaid Services (CMS) via CAP |
| College of American Pathologists (CAP) | 7526035 | CAP |
| New York Clinical Laboratory Evaluation Program (NY CLEP) | 8723 | New York Department of Health |
| California Clinical Laboratory Licence | CDS00800369 | California Department of Public Health |
| Pennsylvania Clinical Laboratory Permit | 32234 | Pennsylvania Department of Health |
| Maryland Clinical Laboratory Permit | 2398 | Maryland Department of Health |
| Rhode Island Clinical Laboratory Permit | LCO01008 | Rhode Island Department of Health |
| ISO13485:2016 | MD674180 | BSI |
| ISO14971:2007 | A28674 | UL |
| IEC62304:2006/ AMD1:2015 | A28674 | UL |
US Laboratory Site (Durham, NC)
| Accreditation / Licence / Permit | Certificate Number | Authorising body |
| CLIA | 34D2102308 | CMS via CAP |
| CAP | 9385996 | CAP |
| NY CLEP | 9177 | New York Department of Health |
| California Clinical Laboratory Licence | CDS00800769 | California Department of Public Health |
| Pennsylvania Clinical Laboratory Permit | 35352 | Pennsylvania Department of Health |
| Maryland Clinical Laboratory Permit | 2984 | Maryland a Department of Health |
| Rhode Island Clinical Laboratory Permit | LCO01091/LCO01415 | Rhode Island Department of Health |
| ISO13485:2016 | MD674180 | BSI |
How our Quality Systems Integrate
Almac Diagnostic Services operate under one unified QMS. The diagram below provides a broad overview of what activities are governed by the relevant regulations and certifications which the QMS complies to.
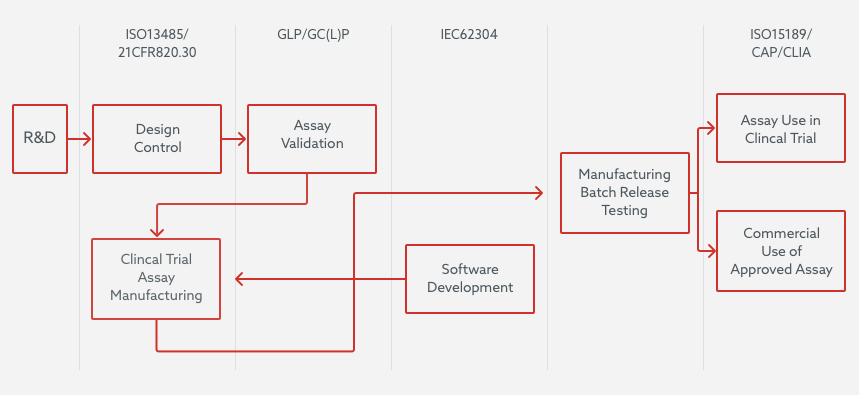
Product Development
For product development, a feasibility/research and development (R&D) phase is generally instigated at the start of a project. Next, the majority of assays are developed as clinical trial assays (CTAs) intended to be used for subject selection/stratification onto clinical trials or to establish the assays clinical performance characteristics. These assays are subject to development under Design Control which meets ISO13485 and 21 CFR 820.30 and ensures they meet the requirements of their intended use. Clinical Trial Assays in addition utilised to analyse samples from trials are analytically validated so that they are fit for purpose for their trial use and meet GCLP principles, and therefore GCP requirements.
Software
In parallel, software developed for the CTA functionality is developed under IEC62304.This ensures that the software is developed under a Quality management system incorporating risk management activities ensuring the extent and scope of the development and testing is based on the based on potential for hazard(s) that could cause injury to the user or patient.
In Vitro Diagnostics (IVD)
Similarly, IVD’s which have been designed and developed under ISO13485/21 CFR 820 and have undergone both analytical and clinical performance evaluation and regulatory approval are manufactured to these standards. They are then subsequently released for commercial use either as a kit to other laboratories or as a functional tested batch of reagents for use on specifically approved instruments in the Almac Diagnostic Services clinical laboratory.
CLIA Regulations
Assays listed on the UKAS scope of accreditation for UK clinical laboratory work are governed by ISO15189. All US and UK clinical laboratory assays, where required by regulation, are governed by CLIA and are accredited by the College of American Pathologists (CAP).
Quality System Compliance
Accreditations
• CAP
• ISO15189
Permits, Licenses & Regulations
• New York (CLEP Permit)
• California
• Pennsylvania
• Maryland
• Rhode Island
Human Tissue Act UK (HTA License)
• GCP
• GCLP
Certifications
(For design and development and manufacturing on in vitro diagnostic nucleic acid technique based assays for gene mutation and expression analysis)
• ISO14971
• IEC62304
• GLP


