Clinical Trial Assay Process
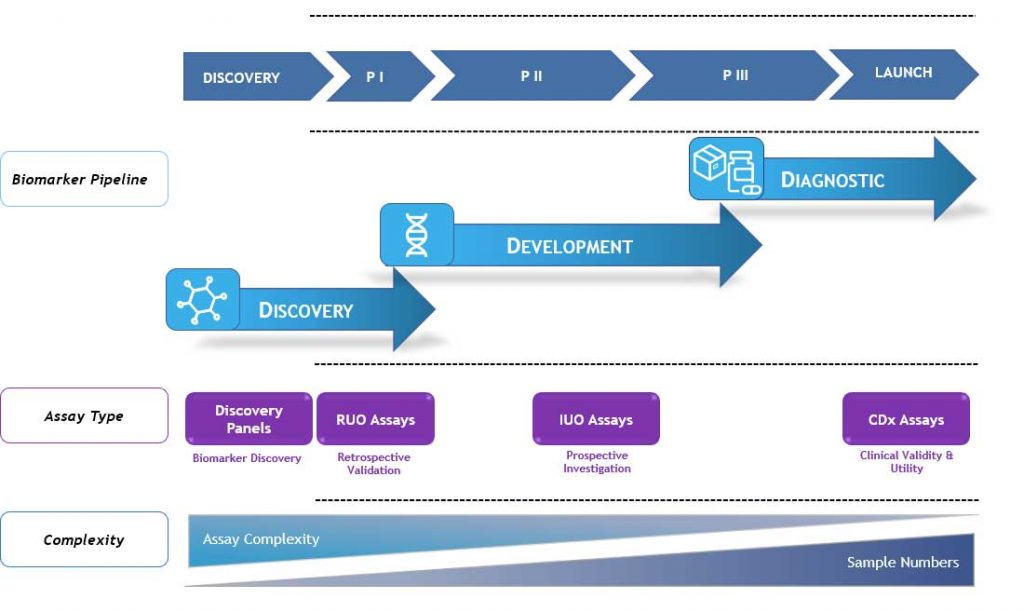
Almac Diagnostic Services offer a complete assay development and validation service that can develop assays across the continuum from RUO to IUO to CDx depending on client biomarker programme needs.
As assays progress from research assays towards assays for use in clinical trial stratification the assay level of complexity increases, as does the stringent validation requirements, regulatory requirements and reporting.
Assay Development
Almac Diagnostic Services has extensive experience in the development and delivery of RNA & DNA based Research Use Only (RUO) assays, generating high quality data for clients.
We work from multiple sample types (FFPE, Fresh Frozen, Blood and Others) and offer clients complete flexibility to be able to run assays on a wide range of platforms including several NGS, qPCR and ddPCR technologies as well as nanoString.
More details on the platforms we offer & our quality and accreditation systems can be found in our Platforms & Technologies and Quality Management System (QMS) sections.
Assay Validation
The process of developing a discovered biomarker into a test suitable for clinical delivery is complex, but it is an area in which Almac has significant experience.
Almac can develop biomarkers into tests for clinical delivery on a range of multiple platforms. We develop, validate and run Investigational Use Only (IUO) assays as part of the clinical trial process where some ultimately progress towards Companion Diagnostic (CDx) assays for commercial launch.
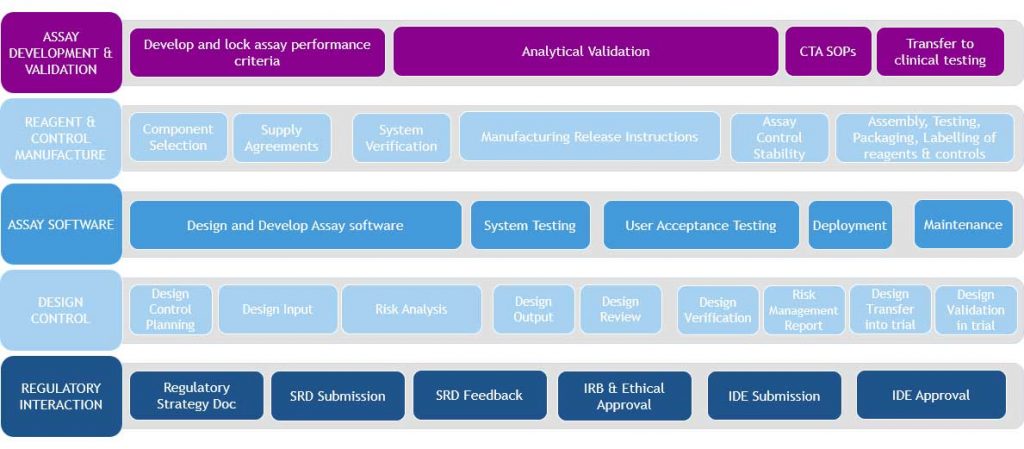
Typical IUO Assay Development Path

Our Range of Services
- Determining the Optimal Platform for Assay Delivery
- Assay Design, Platform Selection & Migration
- Almac Proprietary panel of qPCR reference genes
- Assay Transfer & Validation for clinical delivery
- Control Gene Selection
- Analytical Validation of the assay to CLIA, CLEP, CE-IVD and FDA standards
- Developing the assay instructions for use (IFU)
- Generating appropriate Reference Samples for validation or clinical delivery
- Software (Verified and tested) to automate the production of test reports from raw test data
- Test interface and Reporting
- Phase I, II and III Clinical Trial Testing






