Almac PD Biomarker Testing Service
Almac Diagnostic Services can help Biopharma clients validate reliable PD assays that can help provide invaluable guidance within their drug development programmes.
Almac can develop and perform PD testing on multiple technology platforms across multiple sample types for qPCR and Next Generation Sequencing (NGS) from our global CLIA and CAP-accredited labs.
Clients may wish to provide previous existing data from matched pre- and post-treatment samples and our Data Sciences team can provide solutions for selection of an appropriate PD biomarker, alternatively, clients may already have a pre-defined PD biomarker they would like Almac to test for them.
Almac Diagnostic Services has extensive experience in PD Biomarker Testing. As part of our overall Clinical Trial Solutions we can deliver for clients either:
- An independent PD Biomarker measurement
- A PD Biomarker measurement integrated with a predictive clinical trial assay (CTA)
If a client chooses Almac for combined delivery of both the predictive and PD biomarker within their clinical trial programme Almac can deliver significant efficiencies in terms of time and cost savings.
Specific efficiencies can be gained through centralised sample management, pathology review & nucleic acid extraction.
Benefits
Streamlined Process & Combined Efficiencies
Utilising Almac as a centralised provider, rather than outsourcing PD biomarker work and CTA development work to multiple CRO vendors, creates a much more streamlined process. This also results in logistics efficiencies and reduced risk for client’s drug development programmes.
Cost & Time Savings
This can directly translate into savings for clients with a ‘same sample, same lab’ approach of combined PD biomarker and CTA testing saving time and reducing cost with one provider.
Better Informed Decision Making
Combining drug development data with biomarker efficacy data from Almac focused PD biomarker measurements provides critical information to help inform key drug development milestone decisions much earlier on in the process.


Almac’s unique gene expression report claraTalso allows cancer researchers to discover novel insights within pharmacodynamic (PD) biomarker discovery. By allowing multiple biologies to be evaluated in parallel, the claraT Total mRNA Report supports the identification of pre and post expression changes within novel pathways and biologies that may play a role in response to therapeutic targets.
Example of Almac PD Biomarker Process integrated with a Clinical Trial Assay
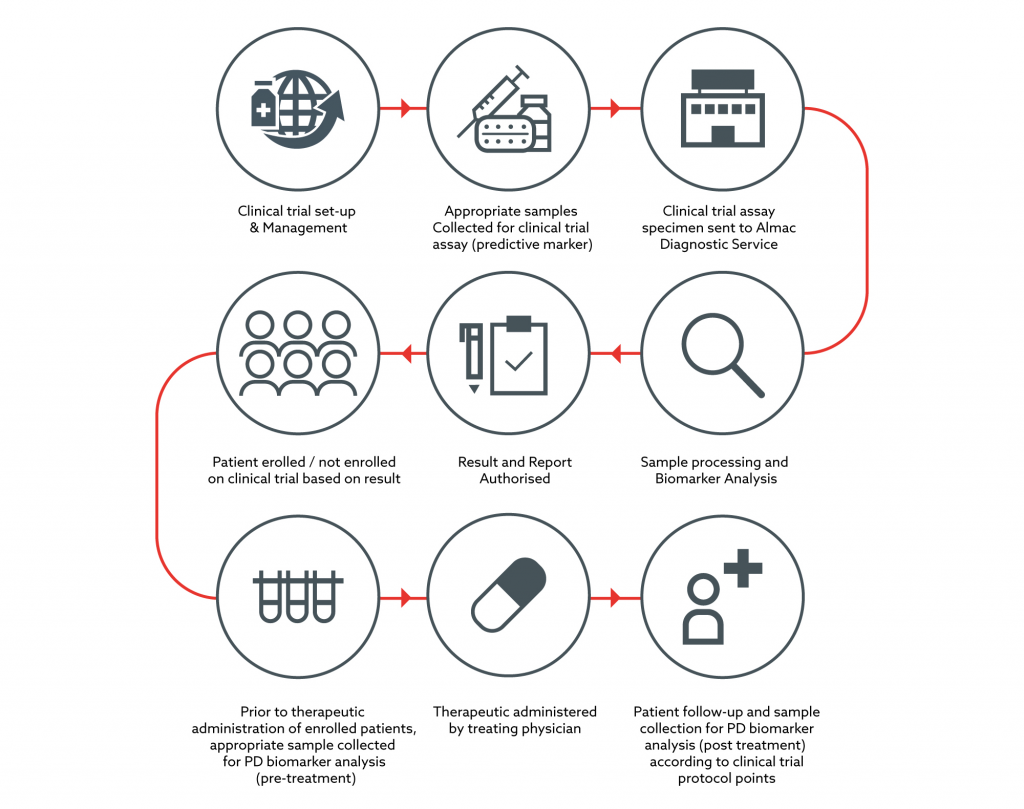
PD Biomarker and predictive CTA combined activity in the same Almac laboratory providing streamlined efficiencies for clients.
Contact Us
Interested in PD Biomarker testing in conjunction with predictive biomarker testing as part of your Clinical Trial? Get in touch with Almac Diagnostic Services for a quote.


