Almac eConsent
Streamlining participant consent for faster, compliant, and more engaging studies
Almac’s eConsent solution is designed to streamline and enhance both electronic and paper-based consent processes. Built for global trials, it ensures regulatory compliance, improves participant understanding, and simplifies the workflow for researchers and participants alike.
Whether used remotely or in person, eConsent supports flexible deployment tailored to your study protocol. Choose from a ready-to-deploy module or full integration with the Almac Trial Coordinator™ ecosystem.
Download eConsent Factsheet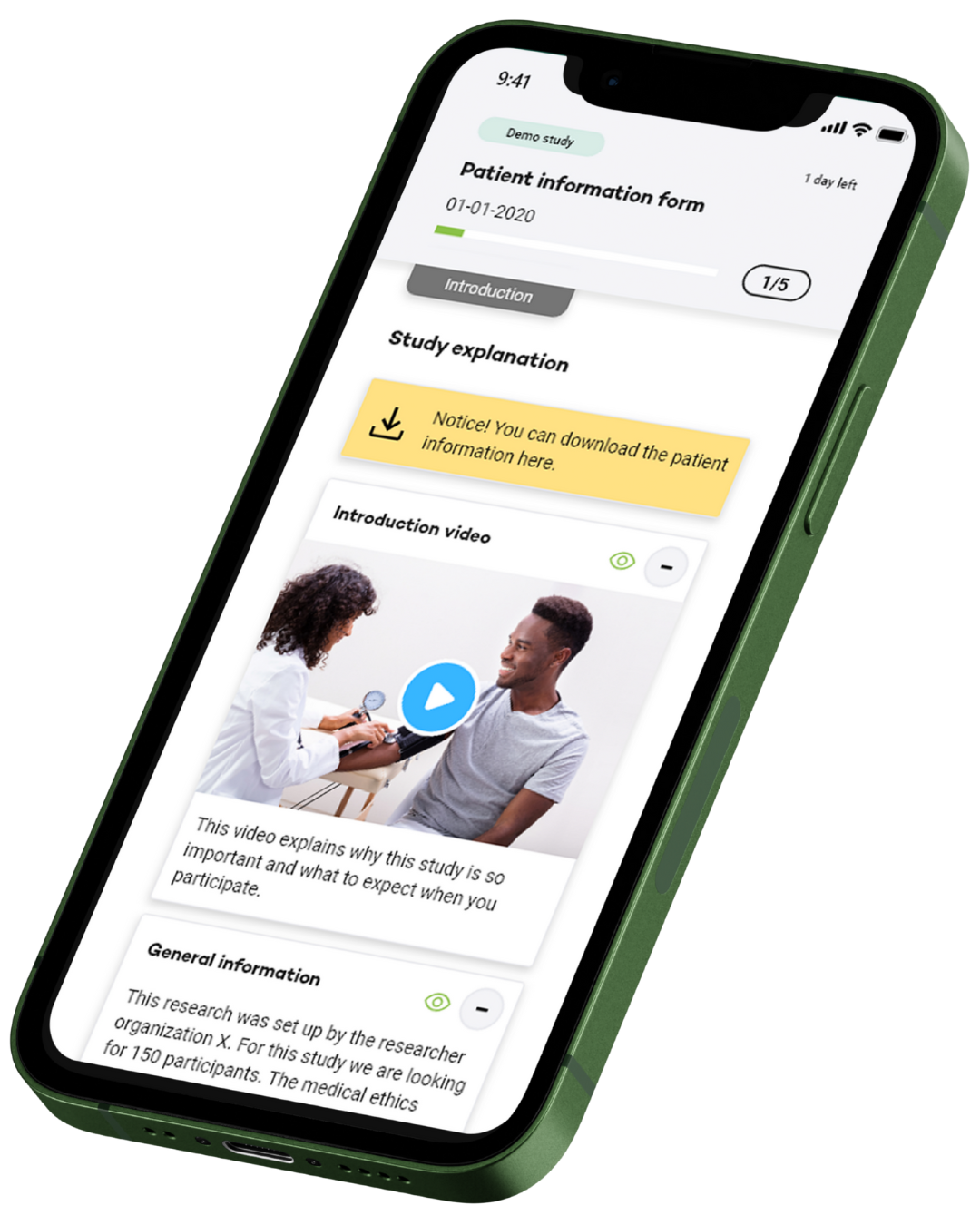
eConsent Key Features
Electronic Signatures with Audit Trails
- Secure, compliant signing with Advanced (AES) or Qualified (QES) Electronic Signatures.
- Full audit trails ensure transparency and meet 21 CFR Part 11 and GDPR requirements.

Real-Time Consent Status Monitoring
- Clinician dashboard provides real-time updates on participant consent status, enabling efficient tracking and timely decision-making.
User-Friendly Interface
- Intuitive design supports seamless navigation for participants of all ages, enhancing engagement and reducing barriers to participation.
Modular & Protocol-Adaptive Configuration
- Customisable to align with study protocols, supporting flexible consent flows and streamlined re-consent processes.
Participant Experience

Compliance for Global Trials
Almac complies with international standards and regulation, ensuring data security and privacy across all operational regions.





Proven Global Delivery
Achieved 98.3% protocol adherence in global oncology trials
Enabled a 42% reduction in missed visits in high-complexity studies
trials supported
countries
sites
Ready to Transform Your Clinical Trials?
Experience the difference with Almac eConsent – streamline data capture, enhance compliance, and empower confident decisions. Contact us to book a demo.

