


CDx Commercialization



CDx Commercialization
CDx Commercialization
Almac Diagnostic Services has the organizational and logistical resources necessary to provide an end-to-end service for CDx commercialization, which can be scaled up to every major market from our clinical laboratory hubs in the USA and Europe & our partnership laboratories in Asia.
Early Commercial Engagement
Almac Diagnostic Services believe that the CDx commercialization activities should be considered as early as possible in the client CDx development process as this will lead to better informed decisions in terms of diagnostic platform choice, chemistry, and regulatory and clinical strategy to ensure no unforeseen commercial barriers prevent the rapid adoption of the test and drug. It allows both BioPharma and Diagnostic company to be completely aligned from the outset so that both parties are clear on the vision and goals for the CDx test.
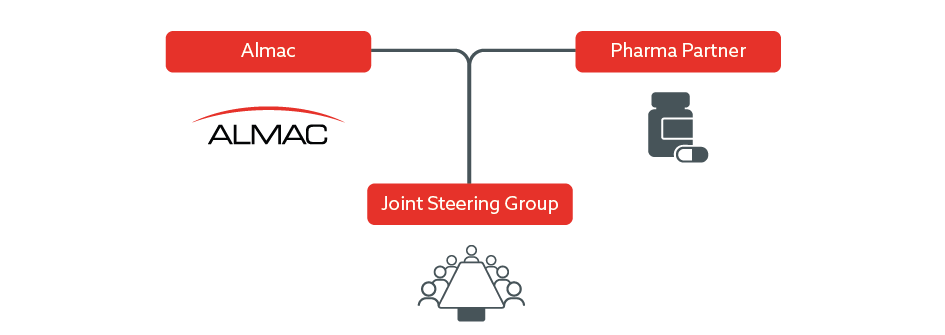
Almac Joint Steering Committee Approach
Almac Diagnostic Services typically establish a joint commercialization committee (JCC) with our partners that meets regularly from the initial kick-off meeting and throughout the CDx development program to agree and implement the commercialization strategy in preparation for launch.
Typical High-Level Tasks:
- Agree Commercial Strategy for CDx Commercialization Plan
- Assigning Roles and Responsibilities
- Agree Communication Plan
- Oversee Execution of CDx Commercialization Plan
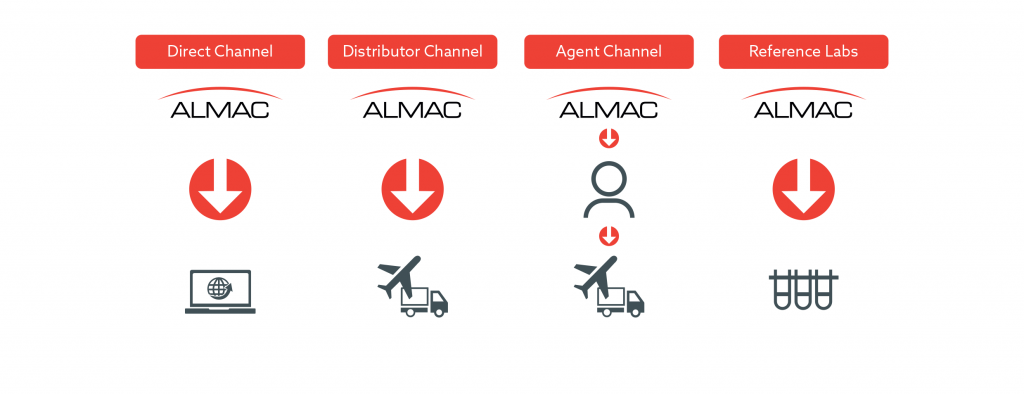
Global Logistics and Distribution Experience
Almac Diagnostic Services has the organizational and logistical resources necessary to provide an end-to-end CDx commercialization service, which can be scaled up to every major market from our clinical laboratory hubs in the USA and Europe & our partnership laboratory in Asia.
Almac Group has 50 years of manufacturing and global distribution experience to support your CDx Commercialization needs.
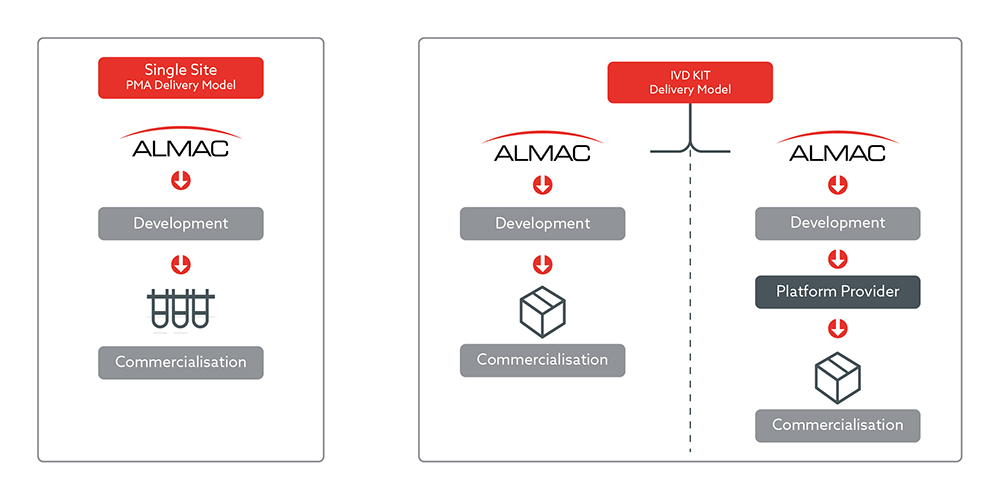
Flexible CDx Commercial Models
Almac Diagnostic Services can offer multiple flexible CDx commercialization models for BioPharma clients to choose from:
- ssPMA Model (Almac)
Where Almac develop and run the test from our laboratory. - IVD Kit Model (Almac)
Where Almac develop and manufacture the IVD test and kit and distribute to other laboratories to run. - IVD Kit Model (Hybrid model)
Almac develop and run the test within the clinical trials initially as a ssPMA and bridge to a platform provider either pre or post launch for commercialization.
Brexit & IVD Regulations- A Unique Almac Advantage
Due to the special status that Northern Ireland has been granted as part of the EU Withdrawal Agreement between EU27 and UK, Northern Ireland will continue to adhere to EU rules on the regulation of medicinal products, medical devices and the movement of goods. This part of the Withdrawal Agreement is known as the “Northern Ireland Protocol”.
The Northern Ireland Protocol will offer Northern Ireland-based companies, like Almac, the opportunity to effectively act as if they are still within the EU with respect to compliance with EU In Vitro Diagnostic (IVD) Regulations and EU Clinical Trial Regulations while still being able to easily access the UK market. In other words, the best of both worlds.
This puts Almac in a unique position to allow clients unfettered and flexible access to support their biomarker clinical trial and CDx development in both the UK & European markets.
Find out more by reading our Almac Voice blog by Dr Stewart McWilliams, Global VP of Quality & Regulatory Affairs at Almac Diagnostic Services

Interested in starting a CDx Partnership with Almac?
Related Resources
Webinar


