Elevating Study Experience in Decentralised Clinical Trials: How IRT Can Help
By Dan Ward
Product Manager
Sponsors are always seeking to make clinical trials faster and to improve the patient and physician experiences. Decentralised trials (or patient-centric trials) have become a critical tool in this pursuit. Limiting a patient’s need to travel to a clinical site has become more common along with the advent of mail-order pharmacies and tele-health visits.
The first successful decentralised trial was completed in 2011 where there was a stronger focus on site-to-patient shipments, mostly occurring outside of the IRT. This trend continued for the remainder of the decade.
With the onset of Covid-19 and ensuing lockdown, there was more emphasis placed on administering a trial with little to no patient in-person visits. Allowing self-administration or with the help of a home health aide trials could continue.
Coming out of the pandemic, we’ll continue to see patients that are now accustomed to more at-home services and DCTs will continue to accelerate enrollment and improve patient satisfaction to administer a trial at home when possible.
Will lead to accelerated enrollment and improve patient satisfaction to administer a trial at home when possible.
There are a lot of vendors in the decentralised space with features outside of the standard IRT. With that, IRTs will need to be able to integrate, provide and utilise data to support protocol goals.
The IRT functions required to support DCT elements should be fully discussed and carefully considered as the protocol is being developed. While much is possible with a sophisticated and flexible IRT system, not everything that’s possible is advisable in each situation. It’s important to be able to distinguish functionality that’s essential from that which is only nice to have or even that which may add unnecessary complexity.
How can IRT add increased flexibility to clinical trials?
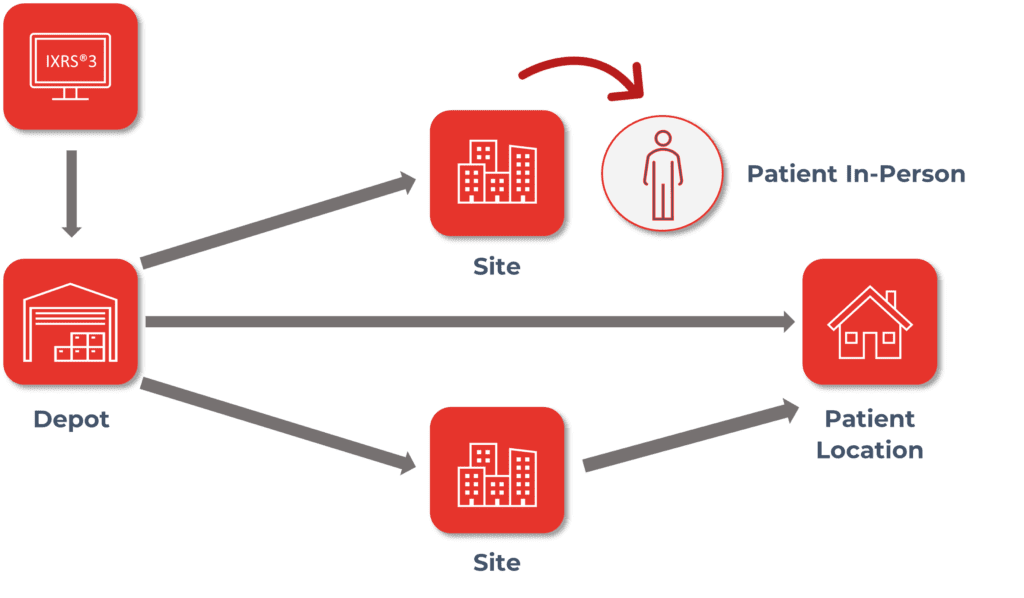
IRTs must provide flexibility to support Direct-to-Patient (DtP) Supply Assignment and Dispensation:
- Assign and Dispense in person at site.
- Assign and Dispense via shipment from site to subject.
- Assign and Dispense via shipment from depot to subject.
There are many variables that come into play with DCTs and flexibility is imperative for the IRT:
- Patient preference – Patients may prefer to see the physician in person, face-to-face for at least some of their visits.
- Protocol necessity – Patients may need to be seen on-site in certain instances for drug administration, assessments, or procedures that cannot be performed remotely.
- Medical necessity – The need to see a patient at the site may change based on the patient’s medical progress.
- The type of investigational product – Not all are good candidates for DtP shipments. Shipping drugs to patients may not be possible with controlled substances or with drugs requiring preparation or strict temperature adherence, to name a few.
- Regulatory requirements – Varying regulations among participating countries may prohibit the use of certain DtP shipments. Depots may be required to have a pharmacist on staff to prescribe investigational product.
How do we ensure patient data remains secure?
Along with flexibility, the ability to coordinate the handling of patient data securely is equally important. GDPR (General Data Protection Regulation) in the European Union and HIPAA (Health Insurance Portability and Accountability Act) in the USA are two of the regulations defining the protection of PII (Personally Identifiable Information). IRTs must manage to allow access only to those entities that need it for Depot-to-Subject shipments. Initially, the approach was to have the site provide PII to the depots directly and not involve the IRT with that data. Moving forward, IRTs can facilitate storage and communication of PII using validated encrypted sources as well.
How can IRT help you manage drug supply?
Supply Algorithms must also be informed on what the configuration is for each subject visit. Projections must take into account what supply will be needed for sites and what supply will be shipped directly to a patient. If in-person visits are required, it should be included in supply projections for the site regardless of the patient’s expected dispensing location. Site-to-patient visits will be supplied the same as an in-person visit since supply will come from site inventory. Visits that are not in-person or site-to-patient should not be included in supply projections for the site. However, supply algorithms may need to allow for additional buffer stock in case a patient who was expected to have supply delivered to their home has supply dispensed in person.
To support the site’s coordination of patients and visits, the IRT must provide as much guidance as possible. IRT should clearly indicate the patient’s expected dispensing location. Site users will need visibility of upcoming visits requiring shipments to patients to allow for supply transit time. Visit registration should allow sites to designate the dispensing location and the expected dispensing location of the next visit. Visit details (historical) should display the dispensing location indicated at the visit. IRT should clearly indicate the patient’s expected dispensing location in reporting and audit documents. Site users should also have visibility to upcoming visits requiring shipments to patients to allow for supply transit time.
What are some common considerations outside of the IRT with DCTs?
- Acknowledgment of Shipment Receipt for a Patient at Home – Site, Courier or Patient are all options for required oversight of proof of dispensing.
- Temperature Monitoring – For patients receiving investigation product at home, viability of investigational product may require a site tele-visit, courier update or home health aide to complete the determination.
- Post-Dispensing Chain of Custody – Accountability, Returns and Destruction of supply shipped to a patient will need to be considered. Patients can return supply to the site to determine consumption and handle return to the depot or destruction.
In Summary
- Decentralised trials, designed and conducted properly, offer improvement of patient centricity by lessening the burden of trial participation, which in turn speeds patient recruitment and strengthens retention.
- The IRT systems that support Decentralised Trials must be properly configured to ensure that patients are treated per the protocol requirements and that all trial processes are efficient and compliant.
- Proper configuration will require system flexibility (the ability to adjust workflows to accommodate all variations of hybrid trials) and the ability to easily integrate with the other technologies that make up the eClinical landscape.






