Almac – Global Clinical Trial Testing
Once a client’s biomarker has been developed into a clinical test, our dedicated Clinical Testing Team can offer Clinical Trial Set up, Clinical Test Delivery and Clinical Trial Management for global clinical trial stratification.
Our Global Laboratories
Our global state-of-the-art CLIA and CAP accredited laboratories based in Craigavon, UK and Durham NC, USA. We also have several partnership agreements in place with labs in China. Find out more about our labs here.
Our Capabilities – At a Glance
| Capabilities |
|---|
| The Clinical Testing Unit is operational 7 days a week: (09:00-17:00 UK Time) |
| Dedicated multidisciplinary team composed of Medical Laboratory Director, Consultant grade Pathologists/Haematologists, Clinical Testing Unit Manager, Technical Supervisor, General Supervisors, Testing Personnel, Laboratory Administrators & Data Specialists. |
| Almac Laboratory adherence to strict regulatory requirements: CLIA/CAP/CLEP/HIPAA/ISO15189
All reagents and process controls manufactured under ISO13485 |
| Strict monitoring of test performance during test delivery with a strong focus on staff competency. |
| Almac offer a fully customised workflow to support clinical test delivery of validated clinical trial assays.
Almac offer a bespoke communication plan for clients including a customised workflow, incorporating: sample receipt workflow, pre-analytical, analytical and post analytical processing, repeat testing workflow, customised patient test reports to meet regulatory requirements, QC metrics and IQC failure checkpoints. |
| Offer a quality-driven service that is both safe and efficient to support the decision process in clinical trial recruitment.
Almac Diagnostic Services has over 15 years’ experience in supporting clinical trial recruitment & testing. |
| Competitive Test Turnaround Time (TAT) monitored as part of the laboratory key performance indicators. |
| Almac employ highly skilled staff across different time zones including a range of pathologists specialising in Cellular Pathology (Histopathology and Cytopathology) & Haematology. |
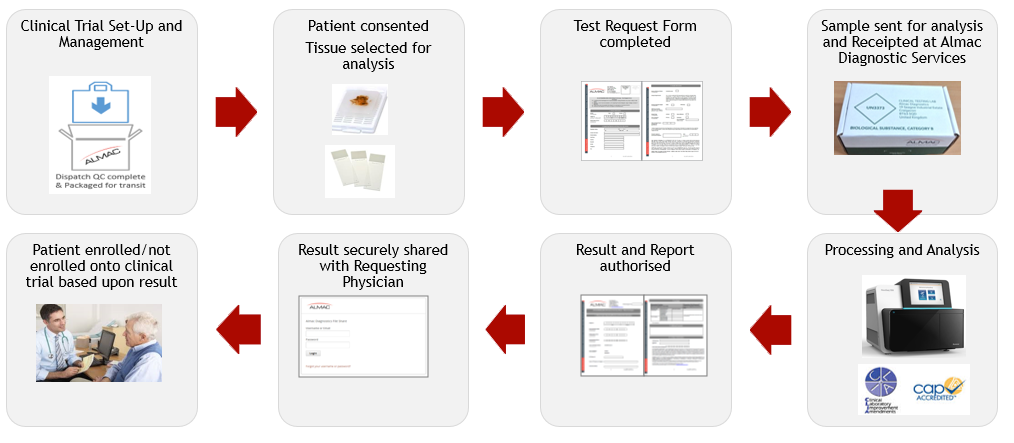
Clinical Testing Process Example:

Clinical Trial Setup & Management service:
Almac Diagnostic Services can also support clinical trial site setup and management including:
- Preparation & distribution of sample collection kits.
- Generation of operations manual.
- Training of clinical sites.
- Communication plan for Clinical Testing.
- Sample shipment and logistics.
Ongoing Clinical Site Management:
Almac Diagnostic Services can also help manage the ongoing clinical site management on behalf of clients to ensure global co-ordination on logistics, training and specimen transport and receipt for clinical studies.
Clinical Site Management
A communication log will be generated and verified to facilitate direct correspondence with the clinical sites to include:
- Liaising with clinical sites regarding sample shipment.
- Answering all receipt, testing and reporting queries.
- Co-ordinating the shipment of additional/replacement samples.
- Co-ordinating sample returns.
Clinical Site Training
- Monitor against expected receipt date.
- Includes working with a large number of multi-national sites.
- Utilise teleconference, on-site seminars, webinars.
Specimen Transport
- Monitor against expected receipt date