Sample Kit Design & Manufacturing
For clinical trials, Almac Diagnostic Services offer a full Sample Kit Design & Manufacturing service alongside logistics capabilities honed over many years in the industry.
Almac’s Range of Capabilities
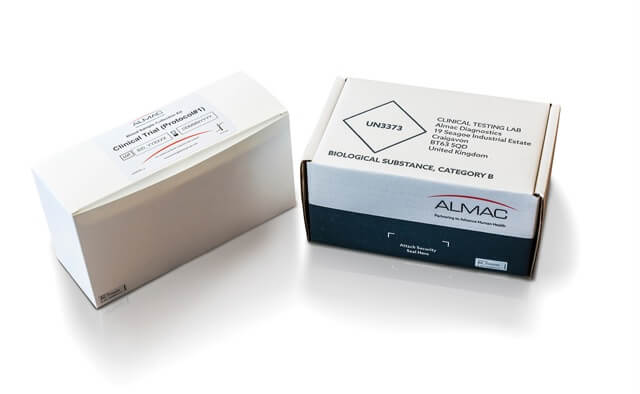


Benefits of Combined Almac Solution
The benefits of using Almac’s Sample Collection Kit Design, Manufacturing & Logistics service in combination with our Clinical Testing capabilities provides clients with better clinical trial outcomes in the following ways:
| Kit Manufacturing & Logistics | Clinical Testing |
|---|---|
| Bespoke Packaging & Label Design service resulting in a well-designed kit minimising site errors during sample collection. | Higher volume of tests ordered accurately Higher percentage of adequate and legible information to proceed with assay testing. |
| Inclusion of bespoke labelling allows easy identification of components, patient samples and kit information. Optimised shipping routes to maintain sample integrity during transport. | Increased Specimen Acceptability Higher percentage of samples meet specimen acceptability criteria to proceed with assay testing. |
| Protocol specific shipping documentation and labelling accelerates the shipping process and reduces shipping errors. Selection of optimised shipping routes to ensure rapid return of samples. Proactive sample tracking to ensure any delays in transport are highlighted and contingencies put in place. | Optimised test turnaround time Higher percentage of samples meet assay test turnaround time. |
| Provision of a dedicated supply chain team to support clinical sites for duration of the clinical trial. | Increased customer satisfaction Higher percentage of clinical sites satisfied with clinical site management and assay communication. |
| Clinical trial inventory management from site initiation ensuring clinical sites have the required kits for first patient in date. | Greater quality control Higher percentage of samples pass quality control and a valid eligibility call reported. |
| Ongoing stock monitoring of sample collection kits throughout the clinical trial. | Enhanced trial setup & trial management service Almac offer a bespoke end to end trial solution designed in line with regulatory requirements for assay testing. |
Sample Collection Kit Design
Almac Diagnostic Services can design and manufacture a range of various bespoke sample collection kits for clients’ clinical trials including the following:
Blood (and blood derivatives including plasma, serum and PBMCs)
- Blood Tubes and Collection Ancillaries
- Blood Transportation Kits
FFPE Tissue
- FFPE Tissue Slides & Blocks
Fresh Tissue
- Fresh Tissue Samples
Liquid Specimens
- Liquid Specimen Collection (Urine, Buccal, Saliva, etc.)
Temperature controlled specimens
- Specimen Shipper (Temperature Controlled)
Examples of Almac Diagnostic Services Sample Collection Kit Manufacture & Design:
Single Image Kits


Bulk Collection Kits
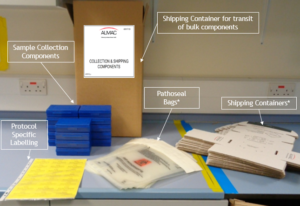

Kit Design examples

Logistics
Almac Diagnostic Services partners with approved logistics specialists with extensive global reach and expert clinical trial knowledge, allowing our Logistics team to advise clients on the most appropriate courier service for their particular clinical trial needs.
We help clients proactively solve potential logistics problems and assist to effectively navigate challenging local import and export regulations. Almac Diagnostic Services dedicated logistics and supply chain team can maximise efficiencies and ensure smooth on-site delivery of sample collection and supplies on time.
Key benefits:
- Almac partnerships in place with multiple specialist global logistics companies.
- Almac global import & export management experience.
- Almac in-house cold chain & dangerous goods specialist.
Brexit Planning
Almac Diagnostic Services currently has processes in place for the import of samples from outside the EU. In the case that the UK leaves the customs union and reverts to WTO rules, Almac will use this process for the import of EU shipments.
- Additional resource has been allocated to ensure all shipments are monitored and to minimise customs clearance entering UK.
- Additional training on customs compliance.
- Almac is working with Almac-approved couriers reviewing shipping lanes for imports and updating if necessary to prevent delays.
- Almac ensure our courier partners can perform import clearance and airport recovery 24/7.
- Contingency planning and preparations have been made to ensure continuity of our service and minimise supply chain risk.
For further information on Almac Group’s Brexit strategy visit our dedicated Brexit Solution Hub here.
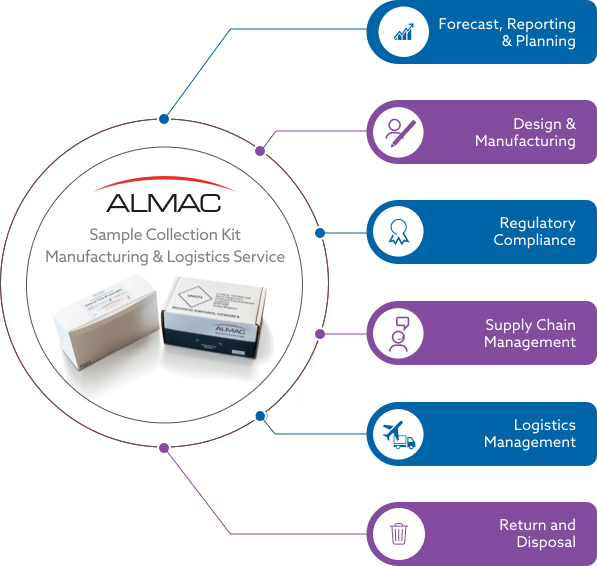
FORECAST, REPORTING & PLANNING
- Dedicated team to meet client trial requirements and minimise costs.
- Bespoke reports for effective trial management.
- Supplies strategy and effective ordering to improve trial efficiency.
DESIGN & MANUFACTURING
- Bespoke Packaging & Label Design service managed by a Design Specialist.
- Technical consultation for component selection.
- Inventory management system and temperature controlled storage solutions
REGULATORY COMPLIANCE
- Adherence to global regulations for import / export and IATA.
- Sample collection kits manufactured under an ISO 13485:2016 certified QMS.
- ISTA and IATA package testing experience.
SUPPLY CHAIN MANAGEMENT
- Dedicated supply chain team to support clinical sites for duration of the clinical trial.
- Clinical site stock management at all clinical trial sites for duration of the study.
- Traceability of manufactured stock at clinical sites.
LOGISTICS MANAGEMENT
- Dedicated logistics team to provide solutions for your trial logistical needs.
- Courier selection and setup based on individual trial requirements.
- Temperature controlled solutions for transport of specimens.
RETURN & DISPOSAL
- Recycling & destruction of expired sample collection kits.


