Almac ONE™: the complete solution for your clinical trial
Only one unified clinical trial supply solution has redefined the supply chain experience to create a responsive end-to end process that bridges the physical and digital connect. The result – enhanced visibility and accuracy throughout the lifecycle of your study.
Collectively, our expertise integrates patient recruitment, clinical supply and Interactive Response Technology (IRT) strategies with closed-loop technology to enable a holistic data flow that leverages insights to give you greater flexibility and visibility over your clinical supply, resulting in enhanced performance – end-to-end.
We call this unified clinical trial supply solution – Almac ONE.
Clinical Supply and Interactive Response Technology Solution
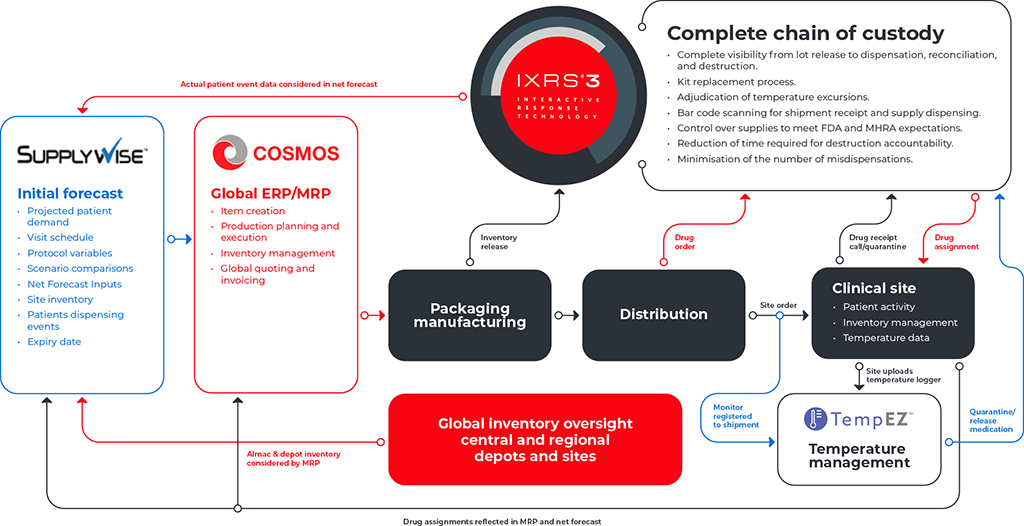
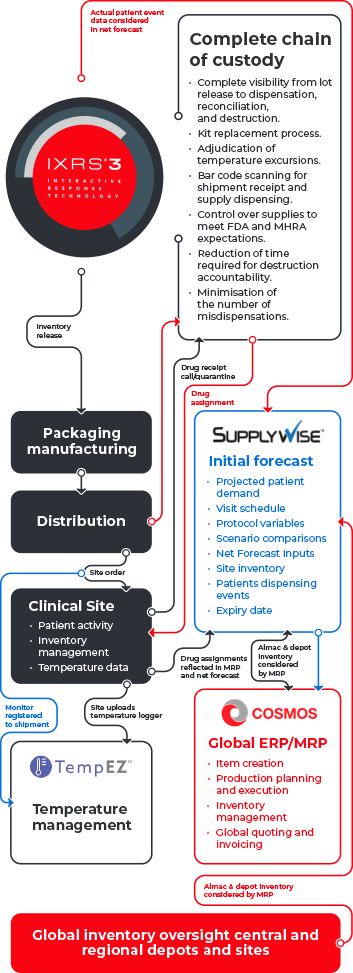
The benefits of a unified clinical trial supply chain
Almac Solutions
Interactive Response Technology (IRT)- IXRS®3
An intuitive and logical way to manage the key components of clinical trials
Find out more
Clinical Storage & Distribution
A global distribution solution to safeguard your patients and clinical trials
Find out more
TempEZ™ Temperature Management
All your temperature data, for all your products, in one place. With you for the journey™
Find out more










